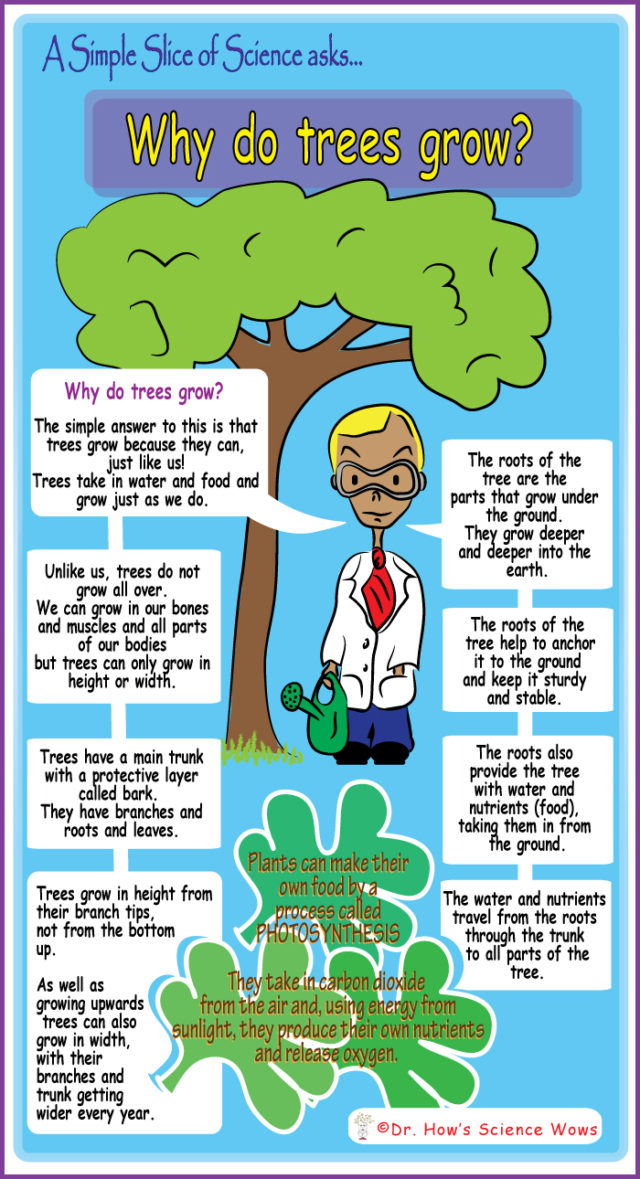
This week’s question for Dr. Simple came in from Eleni who is three and a half! Eleni wants to know …
“Why Do Trees Grow?”
Dr. Simple had to keep himself firmly rooted on the ground to answer this one… I hope this answers your question Eleni!
Science, Curiosity and Life

‘Did you know… trees and plants in rain forests help to make rain; if a forest is cut down the area around it may suffer from drought!’
Transpiration accounts for up to 10% of water in the atmosphere! Some trees can loose hundreds of thousands of litres of water in a single day through transpiration! The water vapour trees transpire into the air cools the air!
 |
| photo credit: Christopher Chan via photopin cc |
Stomata are tiny holes or pores on leaves, stems and even petals of the plant that allow water out of the plant (transpiration) and gases (like carbon dioxide) into the plant. Most of the stromata of a plant are found on the underside of the leaves.
| You will need…. white flowers, clear cups and food colouring of your choice |
| Step 1: Fill cups 2/3 full with water |
| Step 2: Add different food colouring to each one; I used blue, yellow, green, red (Note: you need a fair amount of food colouring… at least half a bottle per cup) |
| Step 3: Cut the stem of four flowers to desired length then add one to each cup |
| Step 4: Leave for a few hours (or overnight) and see what has happened! |
So what has happened?…The coloured water travels up the stem and eventually reaches the flower where it changes the flower’s colour.
What could you change or what other things could you try?… What would you change if you repeated this experiment? You could try it using a variety of different types of flowers to see if one type works better than another. You could vary the amount of food colouring you use.
I had planned a different blog for today but something funny happened yesterday evening and it has lead me down a different path! My children commented on the nice smells from the kitchen and asked me what was for dinner…. “Carbonara” I replied. The two oldest started to chat and whisper to themselves and I was bracing myself for some kind of “I don’t eat that” protest, but instead they just asked the question again…..”What did you say we are having Mum?”. This time they heard my answer more clearly and the two of them cracked up laughing. I couldn’t understand what was so funny until the laughter subsided and they told me…..
……………………………”WE THOUGHT YOU SAID CARBON DIOXIDE!”
This lead to a super nerdy science discussion over dinner about all things carbon dioxide like. The little three year old was very proud of himself telling the others how he made carbon dioxide is his science video with mammy. I have posted this one before but here is a link if you want to see the little Einstein in action!
I was surprised at how much the children knew and how interested they were about carbon dioxide. It is a simple but essential molecule that is an integral part of life on earth.
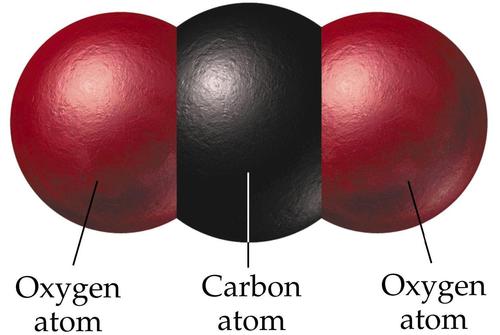
WHAT IS CARBON DIOXIDE?
Carbon Dioxide is a colourless gas. It is made up of one carbon molecule bound to two oxygen atoms and is written as CO2.
 |
| Image source: explainscience.tumblr.com |
WHERE DO WE FIND IT?
Although CO2 makes up less than .04 % of the gases in air it is crucial for the existance of life on earth as we know it. Most of us know that we need oxygen to breath but plants need CO2 for their survival. Green plants take CO2 in from the air and, using energy from the sun, break the molecule down into carbon (C) and two oxygen molecules (O2). The carbon is kept by the plant and converted into starch and sugar. This process is called Photosynthesis. The O2 is then released into the air by the plant.
 |
| Image source: nskamericas.com |
So how does CO2 get replenished back into the air again? This happens through a process called respiration! Animals (including us humans) breath in O2 into our lungs and pass it to every cell in our bodies, through our bloodstream. Our cells need this O2 to grow and to make the energy that powers the body. During the body’s energy making process some of the carbon (from our food) is combined with the O2 forming CO2. This is a waste product in our bodies so it is carried back to the lungs to be released into the air in our breath.
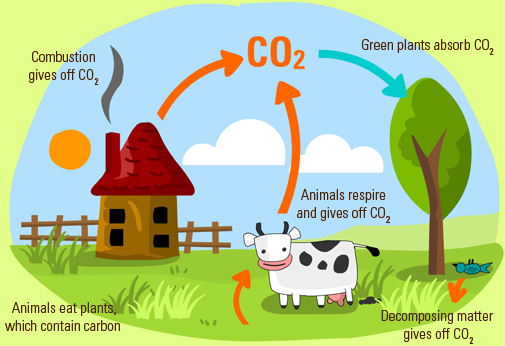
THE CARBON CYCLE
You can start to see a cycle emerging in all this; plants take in CO2 and convert it to food (sugar and starch) and O2. The O2 gets released into the atmosphere. Animals eat the plants and break down the sugar and starch, combining the C part released with O2 forming CO2 again! This is called the carbon cycle.
Other things contribute to the carbon cycle: Carbon is present in our bodies, in fact it is present in all animals, and plants, even rocks and dirt. When bodies and plants die and rot this carbon ends up in the earth. Over time some of this carbon get converted into fossil fuel such as coal, peat or oil.
When we burn these fossil fuels the carbon gets combines with oxygen releasing CO2 back into the atmosphere.
 |
| Image source: eschooltoday.com |
Another step in this cycle is what the plants do at night! In the absence of sunlight plants switch to respiration, which means that, just like us, plants take in O2 and release CO2.
SUBLIMATION
CO2 exists as a gas at standard atmospheric temperature and pressure. It will freeze into a solid state at temperatures below -78oC. Solid CO2 is often called “dry ice”. Dry ice can be used to create a fog or smoke effect and is a great ingredient in many fun science experiments. One of my favourites is making a big bubble using dry ice – I found this demo showing how (click the image below to link to the experiment). If you ever do get some dry ice make sure you work with an adult supervising as it is VERY COLD!
An interesting fact about CO2 is that it does not exist in a liquid state at standard atmospheric pressure. This means that under normal conditions dry ice will turn straight into a gas as it thaws (i.e. it does not go from solid to liquid to gas as most substances do). This process (solid straight to gas) is called sublimation.
GLOBAL WARMING
Some of the gases within our atmosphere are known as greenhouse gases as they absorb some of the sun’s heat that is reflected off the earth and stop it escaping into space. CO2 is one of the greenhouse gases naturally present in our atmosphere.
These greenhouse gases maintain the earth’s temperature and this process is called the greenhouse effect. If the level of a greenhouse gas changes significantly then we experience global climate change. Global warming is the term used to describe an overall increase in global temperature due to an increase in greenhouse gases in our atmosphere. Increased levels of CO2 are thought to be the main contribution to current global warming; These increases are primarily due to the large number of fossil fuels burned by humans and a vast and steady decline of trees globally. Recent reports show a 30 percent increase in atmospheric CO2 levels since the dawn of the industrial revolution. While there is still much debate about the extend of global warming it would appear that consequences such as melting ice caps, rising water levels, global temperature increases, droughts and floods must, at least in part, be attributed to human actions!
Science has turned it’s attention to natural and renewable energies that provide some solutions to preventing and potentially reversing many of the current issues observed with global warming.
AN EXPERIMENT TO TRY
Did you know that some types of fire extinguishers contain CO2? You can make a “mini” fire extinguisher using just vinegar and breadsoda….. but I thought I would let the experts show you!
Further reference:
The carbon cycle
Living in the greenhouse

Ok, this week’s blog inspiration is slightly different to the norm. Usually the idea comes from a question that a child has posed – this time there is a slight deviation, but only slight…. this weeks question came from an adult, but only in the chronological sense – as in fact he is classified as the biggest kid in our house- it was posed by my husband. I was making pancakes the other morning (as I do every weekend on the request of my son) when my husband pondered aloud…”I wonder what the reason behind each ingredient is… and who made the first pancakes”. As I was wondering what next to blog about the two ideas seemed to merge into one, and so was born the question…..
…..”WHAT IS THE SCIENCE BEHIND A GOOD PANCAKE, AND WHO’S IDEA WAS IT?”…..
| Made in the name of science |
A BIT OF HISTORY…
So, before I delve into the science behind the PERFECT PANCAKE, I thought I’d look at a little bit of history first. The pancake as we know it seems to be accredited to the ancient Greeks, who in the 6th century started combining ground wheat with olive oil, honey and milk – and so the first pancake was born. If we expand on our concept of what a pancake really is we could look back further still to the process of making flat bread from ground grains and nuts mixed with milk or water, dating back to the neolithic period.
WHAT DO WE FIND IN A MODERN PANCAKE?
If we start within Europe a modern pancake can be classified as the round flat variety similar to the french crepe which contains some form of flour, and a liquid such as milk or water. These flat pancakes usually also contain eggs and butter, and sugar in the sweetened variety. Then we also have the thicker, fluffier pancakes that contain a raising agent, the name and variation of these include drop scones, Scottish pancakes and of course the well know buttermilk pancakes that are most common in America.
In our house the three most common pancakes made are the buttermilk pancakes, drop scones and the sweet flat crepe like ones.
WHAT GOES INTO A PANCAKE…
If we take a closer look at the primary ingredients we begin to see the complexity and science that really goes into making these delights:
FLOUR… this ingredient can be considered the backbone of the pancake as it provides structure
SUGAR….as well as adding the nice sweet taste and contributing to the colour of the pancake, sugar also keeps the pancake from getting to thick and stodgy
EGGS…. the proteins in the eggs add to the structure of the pancake and to the overall flavour
BUTTER/FAT… as with the sugar, the fats keep the pancake tender and prevent them becoming overly stodgy
MILK/WATER… the liquid portion of the pancake adds to the structure and is necessary for certain chemical reactions to occur
RAISING AGENT… as the name suggests, these agents help raise the pancake, making them light and fluffy
You can of course find many varieties with their own local changes and substitutes, potatoes are commonly used as the starch ingredient instead of flour.
First lets take a look at the thin flat pancake or crepe… in this case we will assume they contain flour, milk and sugar. From the above list we can now predict that the flour is the body of the pancake, it provides the structure, but how does it do this? There are two proteins found in flour called glutenin and gliadin. When moisture is added to flour (in this case the milk) these two proteins link together to form gluten. Gluten is a “sticky” protein, this stickiness allows it to form a network and it is this that adds structure to the batter. Finally we come to the sugar which caramelizes with the heat adding sweetness to the mix and contributing to the colour of the pancake as it cooks. The sugar also prevents the pancakes becoming too thick and stodgy by reducing the amount of gluten produced.
 |
| Image credit: jbeancuisine.com |
So now we move on to the thicker pancakes; the main difference with these is that they contain a raising agent! Yeast is a biological raising agent used in some baking, it produces carbon dioxide gas while digesting sugar and this gas forms tiny bubbles within the yeast. When heat is added during baking these bubbles expand making the bread/cake “rise”.
The main drawback with baking with yeast is that it requires time and who really wants to wait too long for their breakfast? That is why, when using raising agents in pancake mixtures, we substitute the yeast for bread soda and/or yeast; but who can really tell what the difference is between these two?
| Bread soda verse baking powder |
Bread soda (also known as baking soda) is pure sodium bicarbonate. Baking powder contains bread soda but it also contains a powdered acid (usually cream of tartar – potassium bitartrate). Bread soda is an alkali/base and will therefore react with an acid (such as the buttermilk used in pancake batter) producing salt, water and carbon dioxide gas…
BREAD SODA + ACID —–> SALT + H20 + CO2
This carbon dioxide gas gets trapped in thousands of tiny bubbles within the gluten making the pancake batter rise on cooking into light and fluffy wonders! (The same process as with the yeast but a lot quicker).
The baking powder has the added advantage of having the acid already present, so once a liquid is added the dry acid and alkali can react in the same manner as above.
So now that we are starting to understand the science of it all how do we use this knowledge to make the best pancakes. before we jump into this one we first have to consider the science of flavour and odour!
THE SCIENCE OF FLAVOUR
The Maillard reaction describes a chemical reaction requiring certain amino acids and sugars and the addition of heat to produce the molecules responsible for the odours and flavour of food. Now there is a science worth studying!
MAILLARD REACTION: Amino Acids + sugar + heat —-> flavour and odour
So what does this have to do with our pancakes? Well Maillard reactions work best in alkali conditions so bread soda is a definite plus is making golden tasty treats. HOWEVER, add too much bread soda and the pancake will brown too quickly and will have an acrid burnt flavour, not to mention the unpleasant taste produced from the left over breadsoda. It is trickier than we think and yes, of course, someone has already done the science bit for us to work out the ideal amount of bread soda required.
WE ARE HUNGRY – SPEED IT UP
You will be glad to hear that speed is recommended when preping pancakes; Although it is good to allow the batter sit for a few minutes to allow the gluten to “relax” (build up a sufficient network) it has been shown that if left too long the bubbles will have burst and the pancakes will be flat and dense once cooked.
SCIENCE IN MY KITCHEN
I decided I had to try some of this pancake science out for myself so turned to my original buttermilk pancake mix from the wonderful NIGELLA LAWSON. This recipe actually uses both baking soda and baking powder (I omitted the banana). I decided to test out two theories…
1. Does the amount of bread soda determine the colour and flavour of the pancake?
2. Does the length of time the batter is left standing really make that much of a difference?
To keep it simple, I decided to keep everything else (including the amount of baking powder) constant.
So I donned my apron in favour of my labcoat and I set to work. I prepared the basic batter mix excluding the addition of bread soda. To digress for a moment, I also followed another golden pancake rule – not to over-mix the batter (a few small lumps of flour allows it better absorb the liquid and produce gluten).
| My “slighlty lumpy” pancake batter |
I dived my basic batter mix between four bowls and then added different amounts of bread soda to each (the first bowl had no bread soda, the second had half the recommended amount, the third had the recommended amount and the fourth had double what was recommended!). Then I let the batter sit for five minutes before cooking the pancakes.
Here are my results… the pancake on the top left had no bread soda, top right had 1/2 the recommended amount, bottom left had the ideal amount and bottom right had twice the recommended amount. You can see how the pancake gets darker with the addition of more bread soda, with the last one being just a bit too dark. The taste test revealed that the one on the bottom left had the best taste (and texture) and that the one with the most bread soda had that unpleasant taste of bread soda!
To investigate my second question I left the same pancake batters sit for two hours before cooking them. As you can see the pancakes cooked after two hours were indeed a lot less light and fluffy and were a bit soggy inside!
| The batter for these was left sit for five minutes |
| The batter for these was left two hours |
WHY ARE PANCAKES ROUND?
Pancakes are round for two main reasons: gravity and surface tension. Assuming that the pancake pan is flat then once the batter is added gravity will pull on all parts of the batter uniformly in all directions, pulling it out into a round shape. Surface tension pulls evenly on the edges keeping them restrained into the round shape.
THE SCIENCE OF FLIPPING A PANCAKE…
Would you believe that someone has actually looked into the exact science of pancake flipping? How cool is that ….
According to University Professor of Mathematics Frank Smith, the simple mathematical formula for the perfect flip is: L = 4 H /P- D / 2
(L = hand distance from inner edge of the pancake / H = height of flip / D = diameter of pancake)
If that sounds a bit too complicated check this out …
Dr. Tungate, a senior physics lecturer at Birmingham University, found that “a pancake should be flipped into the air at a speed of 10 miles-an-hour, which means that it takes less than .5 of a second to reach the top of its trajectory.”
AND THEN THERE IS THE SCIENCE OF WHAT YOU ACTUALLY PUT ON YOUR PANCAKES…
… but I think that is a whole other blog! So whatever toppings you choose I hope you enjoy your pancakes today!!
| All that science made me hungry! |
And if you still want more….HERE IS AN EXPERIMENT YOU CAN TRY….
Inflating balloons… This experiment shows two fun ways of inflating balloons, kids will love it, it’s easy to do and it teaches some kitchen science… like the difference between using yeast and bread soda as raising agents in baking!
Further reading:
Pancakes served with a side of science.
Celebrate your pancakes with a side of science.
Baking powder verses baking soda.
The history of pancakes.